In the shadow of the worldwide healthcare market is a paradox that contradicts all traditional market logic: 300 million patients around the world suffer from approximately 7,000 rare diseases, but 95% of them have no approved treatment to help them. For years, this crisis was written off as an economic “problem that can’t be solved,” a “market failure” in which traditional drug development models cannot support themselves with fragmented patient populations and unsustainable stakes.
But the narrative is shifting. Artificial intelligence isn’t just speeding up rare disease research; it’s reframing its economics. By putting data scarcity into actionable information, AI is breaking down the barriers that imprisoned these patients in therapeutic purgatory. This article breaks down how machine learning, collaborative data ecosystems, and AI-driven precision medicine are helping to convert orphan diseases into real targets — and why this is the biggest moonshot for pharma since the genomic revolution.
The Rare Disease Paradox: Why Traditional Pharma Economics Fail
In the merciless calculus of drug development, rare diseases are a category of heartbreaking irony — collectively devastating, even if each disease is too small on its own to attract the attention of the marketplace. This paradox is both the fundamental paradox and the unresolved paradox at the centre of modern medicine’s most enduring blind spot.
The Magnitude Gap: Scale, Not Market Power
The numbers paint a grim picture: There are over 300 million people in the world living with more than 7,000 identified rare and genetic diseases, a group larger than that of the total population of the United States. But that huge collective burden fractures into thousands of small pools of patients, each so small that they can’t command any market attention. Just 5% of these conditions have treatments that are approved by the F.D.A., which means that tens of millions remain in therapeutic limbo.
What looks like neglect is, in fact, the market in action. When a disease affects fewer than 200,000 Americans (the F.D.A. “orphan” threshold), it doesn’t have enough of a critical mass to attract pharmaceutical investment. This is not just a matter of callous economics — there is a fundamental mismatch between industrial drug development into biological reality.
The Triple Economic Barrier
Rare diseases bring three interlocking pressures to bear on the traditional pharmaceutical model:
A. Patient Recruitment Crisis
The challenge of rare disease trials is a statistical irony: they require strong patient cohorts to show convincing efficacy, even though rarity is precisely what defines them.
For a disease affecting 1 person in 100,000, the recruitment of 50 patients would necessitate a screening process that goes well beyond that of normal clinical practice. When the patients are scattered across multiple continents, phenotypically diverse despite sharing the same mutations, and are frequently misdiagnosed for many years, recruitment becomes almost impossible. The things that may take 18 months in diabetes can take 5+ years in rare diseases—if you can enrol.
B. Cost-Return Imbalance
The cost of developing a drug is approximately $2.6B according to (Tufts CSDD); this figure conflicts with rare disease revenue caps.
Brutal Math: A drug for 10,000 people at $300,000/year (which is standard for gene therapy) makes $3B a year — just about recouping its R&D costs if it cures every individual (which it never does).
C. Risk Concentration
Rare diseases have failure rates higher than 90% because of:
- Poorly understood disease mechanisms
- Heterogeneous patient responses
- Lack of predictive biomarkers
- Investors don’t like taking on the capital risk for such a small market.
The Orphan Drug Act: Noble Intent, Structural Limitations
In 1983, the Orphan Drug Act tried to correct this market failure with specific incentives:
- Seven years of market exclusivity (instead of the usual 5 for patents)
- Tax credits on clinical investigation costs (up to 50%)
- Waived FDA user fees
Streamlined regulatory pathways
The incentives have been effective — more than 1,000 orphan drug approvals have been granted since 1983, compared to 10 in the decade prior. Yet they do not address three vital shortcomings:
- “Niche Picking”: Businesses forgo ultra-rare diseases (affecting fewer than 1,000 patients) in favor of rarer diseases with larger patient populations (e.g., cystic fibrosis).
- Pricing: Exclusivity leads to a stratospheric price tag ($4.25 million per year for Lenmeldy™) with no data to match.
- No Solution for Trial Design: Incentives don’t solve the fundamental problem:
How to prove efficacy in tiny and scattered populations?
AI’s Game-Changing Role in Rare Disease Research: Rewriting the R&D Playbook
The standard model under which drugs are developed for rare diseases is not simply inefficient; it’s hopelessly broken. AI is disrupting an entire paradigm by directly targeting 4 bottleneck breakthroughs:
1. Target Discovery: From Years to Hours
The Crisis: Finding druggable biological targets used to require 3-5 years of trial and error biology at a cost of $400M-$1B per target. For even orphan diseases where the pathway is not well-characterized, that stage was usually the bottleneck.
AI’s Breakthrough:
- AlphaFold Revolution: DeepMind’s protein-folding AI surpassed 200M proteins—including thousands associated with rare diseases—in 18 months. What structural biologists used to take decades now happens in months.
- Causal AI > Correlation: platforms like BenevolentAI’s “Knowledge Graph” mesh together hidden relationships between >1B biomedical data points to identify targets that are causally linked to disease (e.g., uncovering PDE10A as a target for chorea-acanthocytosis).
Impact: Target ID time reduced from years to <6 months, decreasing costs by 80%.
2. Drug Repurposing: Rescuing Shelved Assets
The Crisis: 93% of rare diseases have no chemical starting point. It costs ~$2.6B to bring a De novo to market in 12 years—far too expensive for small markets to bear.
AI’s Breakthrough:
Transcriptomic Signature Matching: Systems such as the Broad Institute’s Connectivity Map (CMap) use machine learning to match gene expression signatures specific to diseases against the inverse expression profiles generated by existing drugs. This “signature reversion” strategy revealed edaravone (already in use for stroke) as a treatment for ALS which was approved by the FDA.
Cost Collapse: Cost for successful repurpose: $80-$120M (vs. $2.6B for novel drugs), taking 3-5 years.
3. Predictive Modeling: The Death of Big Cohorts
The Crisis: It can take 5+ years to enrol 50 patients for a P2 rare disease trial. [30% of trials are unable to be completed due to under-enrolment.
AI’s Breakthrough:
Digital Twins: Companies like Unlearn.AI design computable patient entities modelled on observational data. These “twins” are synthetic control arms which will lead to a 50-70% in patient numbers.
Impact: Trials for ultrarare diseases (e.g., fibrodysplasia ossificans progressiva) can now be conducted with 15-20 patients instead of 100+.
4. Virtual Screening
The Crisis: High-Throughput Screening (HTS) Each HTS screen tests 1M+ compounds at $500K – 1M/week with <0.01% hit rates.
AI’s Breakthrough:
Generative Chemistry: Models such as Insilico Medicine’s GENTRL are used to design new molecules in silico by maximizing their binding affinity.
Real-World Result: Verge Genomics identified an ALS lead by computationally screening 11M compounds against AI-predicted targets—no wet-lab work until well into lead optimization.
Data as the New Currency: From Scarcity to Abundance
The time of data starvation in healthcare is quickly transforming into an era of data overfeeding, which is shaping the approach towards precision medicine.
Playing a key role in this shift are patient registries and biobanks, which gather large amounts of genetic data. Projects such as the UK Biobank and the Global Alliance for Genomics and Health (GA4GH) have gained sufficient momentum to allow scraping the world’s genetic data for rare disease mutations, uncovering genotype-phenotype correlations, and finding hidden biomarkers.
Adding to this are the abundance of electronic health records (EHRs) that unlock undisclosed patterns amongst widespread populations using advanced natural language processing and machine learning techniques. EHR mining has revealed, for instance, subtle early-warning signs of diseases such as Parkinson’s and ALS that trigger interventions years before current diagnostic schedules.
Additionally, there is a further revolution in the way we track health, through RWD (real-world data) generation through wearables and remote monitoring technologies. Devices such as Apple Watch and BioStamp nPoint record continuous streams of physiological data in real-life scenarios, giving rise to data volumes that are larger in scale and more ecologically valid than clinic-based measurements. These instruments are already revolutionising clinical trials by enabling treatment efficacy and safety to be monitored in real time, reducing the need for occasional in-clinic visits.
Similarly disruptive are patient communities and social networks, which are providing vast amounts of phenotypic data and RWE. Some platforms, such as PatientsLikeMe and RARE-X, allow for never-before-seen data generosity as people generously share in-depth symptom pictures, responses to treatment and the day-to-day reality of their lives. This information is an incredible complement to traditional clinical studies, revealing findings on how disease evolves and on drug response, which conventional trials are unable to capture.
With the evolution of the data ecosystem from scarcity to abundance, the next hurdle is to combine these various sources of data into coherent, interoperable systems. Those institutions that come to perfect that synthesis—integrating genomics, electronic health records (EHRs), wearables, and patient networks—will not just speed therapies to cure, they will reimagine the architecture of health innovation itself.
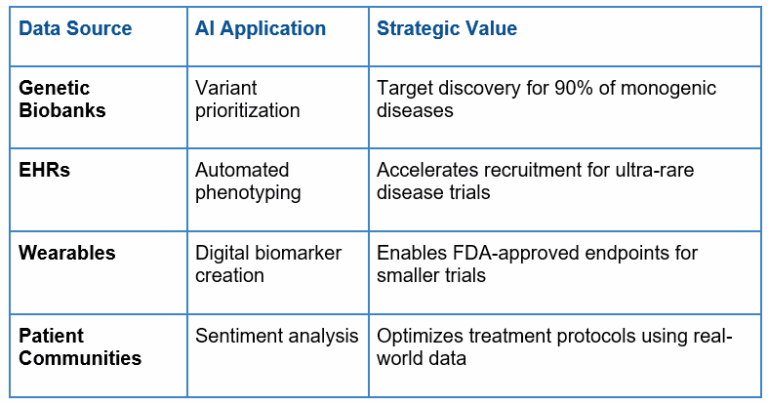
The Integrated Power of Big Data
The combination of genetic biobanking, RWD, wearables and patient communities is generating a virtuous circle of data generation and application:
Navigating Unique Challenges in Rare Disease AI
The application of AI to rare diseases has special challenges, which require proactive, innovative solutions, from data paucity and phenotypic diversity, through regulatory obstacles to representation in datasets. In doing so, rare disease AI is not just improving the lives of underrepresented communities but also setting the standard for the future of healthcare innovation.
1. Advanced Modelling Approaches: Overcoming Data Scarcity
Rare diseases are characterized by small patient populations, and data scarcity is a primary concern. Conventional machine learning methods perform poorly because of the lack of training data, which means the use of complex modelling techniques is required. Of these, transfer learning has become more and more indispensable, in which AI systems can utilize the expertise of equivalent well-known diseases.
For instance, DeepMind’s AlphaFold was originally trained on common proteins but has been repurposed to predict the folding structures of rare, mutation-specific proteins. Similarly, synthetic data generation (by generative adversarial networks: GANs) is providing virtual cohorts that emulate the patient profiles of those with rare diseases, enabling researchers to perform thousands of simulations without compromising patient privacy. These techniques are fundamental to train and test AI models within data-starved environments.
2. Addressing Heterogeneous Disease Presentations and Comorbid Phenotypes
In most rare diseases, symptoms present heterogeneously, with patients having signs and symptoms that do not always match those described in the literature. AI is particularly good at recognizing subtle phenotypic signals, sometimes beyond the capacity of experienced clinicians.
For example, the multimodal AI model combines multimodal data types (genomics, imaging, clinical notes, patient-reported outcomes) to provide a more comprehensive description of rare diseases.
The philosophy of the NVIDIA Clara federated learning platform is an example of this approach: hospitals around the world are linked together to analyze rare disease cases without pulling sensitive patient data. Synthesizing multi-omic profiles and clinical features, these tools can separate overlapping phenotypes and indicate distinct disease subtypes, as observed in rare neuromuscular disorders such as Charcot-Marie-Tooth disease. Crucially, such AI-derived insights are not only advancing diagnostic precision but also offering personalised therapeutic approaches particular to patient-endophenotypes.
3. Regulatory Considerations for AI-Generated Evidence in Small Populations
The regulation of AI for rare diseases research is developing to meet the distinct challenges of small groups. Conventional evidence generation paradigms are based on a series of large randomized controlled trials; however, such an approach is usually impractical in rare diseases.
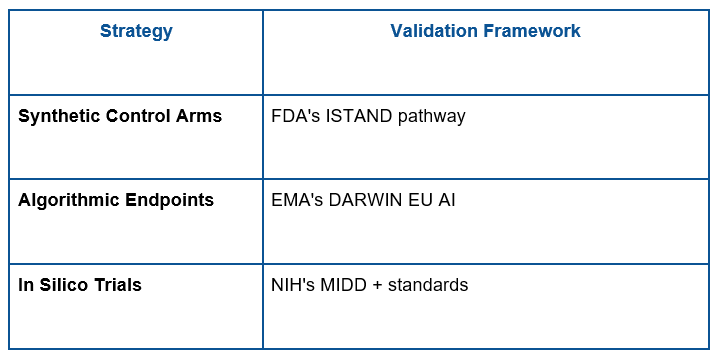
Regulators such as the FDA and the EMA are becoming more open to adaptive trial designs and AI-generated evidence, including synthetic control arms and in silico trials. For instance, the FDA AI/ML Action Plan will permit the use of diverse real-world data and dynamic modeling to help make approvals in small populations.
Regulators are also asking for visibility and auditability in AI models, where sponsors need to show how decisions are reached, and algorithms must be validated with external data sets, however. These standards in development guarantee that AI-generated evidence can maintain the same level of rigor as traditional approaches while being flexible to the unique challenges of rare diseases research.
FDA/EMA-Endorsed Solutions:
4. Ensuring Demographic and Geographic Diversity
A challenging issue in rare disease AI is the absence of demographic and geographic diversity in databases. Research into rare diseases has traditionally focused on high-income countries, where people living in low-resource settings continue to be underrepresented.
This bias could lead to furthering health disparities and curb the real-world applicability of the AI models. Efforts like NIH’s All of Us Research Program and GA4GH are working to address this need by diversifying biobanks and patient registries. Federated learning also enables AI training and local populations to contribute to and benefit from progress without sharing raw data, which would be beneficial for organisations in low-resource settings.
For instance, a recent application in sub-Saharan Africa applied federated AI to sickle cell disease patient cohorts, revealing genetic modifiers that were missed in Western datasets. Guaranteeing diversity does not just deliver a sturdy AI model: it democratises rare disease diagnostics and treatment.
New Economic Models: Making Small Markets Viable
AI, precision medicine and platform technologies are changing the economics of rare disease, transforming those small markets from impossibly uneconomic to investable. Artificial intelligence-led development approaches deliver dramatic cost savings by hastening target identification, trial designs and regulatory filings.
For instance, the use of AI-manufactured virtual control arms can reduce the size of patient cohorts required by up to 50%, reducing the cost of a trial. Furthermore, wearables data in real time and adaptive trial designs make the best use of resources and can reduce ~40- 60% in time-to-market. These developments make ultra-rare diseases economically viable to develop interests in even when patient populations are less than 1,000.
Precision medicine strategies and platform technologies can improve success rates and scalability. Industry can focus on well-defined patient subsets using molecular subtyping and biomarker-driven trials that can result in success rates up to 30% higher.
Modular platforms, including mRNA and gene therapy systems, can scale quickly and the technology can be rapidly adapted to many diseases, while reducing per-condition cost by 60-70%.
In addition to this, outcomes-based pricing mechanisms have payments tied to actual patient outcomes, which provide payers with cost predictability while guaranteeing access to costly orphan drugs.
For example, outcome-based contracts, instalment-based payments and risk-sharing agreements mean that therapies produce measurable effects. All together, these advances are turning rare disease from a high-risk venture into a strategic opportunity that is reshaping the economics of the pharmaceutical industry.
The Patient-Centred Revolution
AI in healthcare is redefining patient experiences, especially for rare diseases, by tackling the “diagnostic odyssey” of care. It used to take 5-7 years for patients to get a diagnosis, during which time they would suffer from misdiagnoses and unnecessary treatments. With AI-powered diagnostics, we are now compressing this timeline to weeks by analyzing multi-omic data, EHRs and imaging to diagnose rare conditions with unprecedented accuracy.
For instance, deep learning models such as Fabric GEM and Face2Gene recognize genetic syndromes within minutes using facial phenotypes, and AI-enabled NLP tools parse through an individual’s clinical notes to identify subtle patterns of symptoms. Such developments also minimize diagnostic delays and permit earlier interventional therapies, which likely have a favourable impact on patient prognosis and quality of life.
The era of personalized treatment protocols based on an individual’s genetic and molecular make-up is revolutionizing precision medicine. AI-based algorithms combine genomics, proteomics and patient-reported data to suggest personalized treatments that would be most effective with the least side effects.
For example, gene-editing therapies for rare diseases, such as sickle cell anemia, that rely on CRISPR technology are customized to a patient’s individual genetic code and have transformative effects.
Furthermore, decentralized trials powered by wearables or telemedicine platforms are making it possible to recruit a more diverse range of patients regardless of geography. These types of trials remove the need for travel and clinic-based monitoring, making them accessible to the underserved and at the same time providing the opportunity to generate real-world evidence to refine treatments.
In addition to clinical progress, these breakthroughs are changing patient pathways from those of relative isolation to ones of empowerment. Patients with rare diseases, who frequently felt alienated from mainstream health care systems, are finding a voice through decentralized trials and patient-led efforts to share their data, such as RARE-X and PatientsLikeMe.
These platforms support patient-generated intelligence and enable the patient to feel empowered in health. AI tools also take this empowerment further by generating actionable information for managing diseases, thus enabling the patient to make more informed decisions regarding their care. The combined change marks a deep reimagining of health care-one in which patients are no longer mere beneficiaries, but active protagonists in their medical destinies.
Future Horizons: The Road Ahead
Advances in technology will change the speed and size of research into rare diseases. AI-Quantum computing convergence, showcased by tools such as IBM Quantum and Deepcell, will make molecular simulation and biomarker discovery work 100× faster, resulting in the emergence of new drugs at warp speed, 100× faster than what’s happening today.
Spatial omics technologies like NanoString’s CosMx will do so at a level of resolution that has not been possible before, identifying new therapeutic targets. Yet, such advancements require sturdy infrastructure – petabyte-level data storage, federated learning systems to consolidate global data, and legal frameworks to inform real-time AI adoption.
Ethical and policy frameworks need to keep pace with the rapid pace of innovation to ensure shared benefits. There should be a strict review of bias in AI algorithms and requirements on what the composition of training data-sets should be (it needs to fairly represent the target population) to ensure that there is no bias in diagnosis and treatment access.
Initiatives such as expedited AI approval programs (modelled on the FDA’s Breakthrough Devices Program) and incentives for rare disease data pools in the public domain could help fuel innovation while maintaining accountability.
At a global level, projects like the WHO’s Rare Disease Data Sharing Accord should take the lead in making interoperability and patient ownership of health data a priority. Rare disease research isn’t just a frontier of innovation — it’s a proving ground for a healthcare ecosystem that’s moral, equitable and can sustain itself.
Conclusion
AI has changed the trajectory of rare diseases from forgotten diseases to innovation powerhouses. From speeding up diagnoses, to tailoring therapies, and encouraging collaboration across borders, by making even these rarest of diseases no match for science superheroes.
To maintain momentum, all key stakeholders need to come together: industry leaders need to put data transparency and shared innovation at the top of their agendas, regulators need to clear the way for AI-driven therapies and patient advocates need to demand equitable access to breakthroughs.
The message is stark – rare diseases are a moral and economic challenge, opening up billions in healthcare value, while bringing back hope to millions of patients. The future is closer than you think: a world where no disease is too rare to be treatable, and precision medicine turns lives once deemed impossible into a new human future every day.
Found this article interesting?
1. Follow Dr Andrée Bates LinkedIn Profile Now
Revolutionize your team’s AI solution vendor choice process and unlock unparalleled efficiency and save millions on poor AI vendor choices that are not meeting your needs! Stop wasting precious time sifting through countless vendors and gain instant access to a curated list of top-tier companies, expertly vetted by leading pharma AI experts.
Every year, we rigorously interview thousands of AI companies that tackle pharma challenges head-on. Our comprehensive evaluations cover whether the solution delivers what is needed, their client results, their AI sophistication, cost-benefit ratio, demos, and more. We provide an exclusive, dynamic database, updated weekly, brimming with the best AI vendors for every business unit and challenge. Plus, our cutting-edge AI technology makes searching it by business unit, challenge, vendors or demo videos and information a breeze.
- Discover vendors delivering out-of-the-box AI solutions tailored to your needs.
- Identify the best of the best effortlessly.
Anticipate results with confidence.
Transform your AI strategy with our expertly curated vendors that walk the talk, and stay ahead in the fast-paced world of pharma AI!
Get on the wait list to access this today. Click here.
4. Take our FREE AI for Pharma Assessment
This assessment will score your current leveraging of AI against industry best practice benchmarks, and you’ll receive a report outlining what 4 key areas you can improve on to be successful in transforming your organization or business unit.
Plus receive a free link to our webinar ‘AI in Pharma: Don’t be Left Behind’. Link to assessment here
5. Learn more about AI in Pharma in your own time
We have created an in-depth on-demand training about AI specifically for pharma that translate it into easy understanding of AI and how to apply it in all the different pharma business units — Click here to find out more.