The pharmaceutical industry is fighting a silent, devastating epidemic, with counterfeit drugs pouring into global supply chains at an alarming and unprecedented rate. With counterfeiters using advanced production methods that can copy even the chemical and packaging structure of genuine items, the industry is fighting an uphill battle to preserve the trust the therapeutic community has in it.
Traditional safeguards such as serialization, batch tracking, and visual inspection are alarmingly insufficient, exposing even the most regulated of markets to breaches that endanger patient lives, damage brand equity and incite expensive recalls.
Considering the global drug supply, only a small fraction is inspected by regulatory bodies; therefore, a new approach is imperative. Artificial intelligence represents the current investment that has the potential to reset the pharmaceutical defence. Through the power of machine learning, computer vision, and predictive analytics, AI makes something possible that was once unimaginable: Real-time detection of counterfeits, analysis of supply chain vulnerabilities at scale, and the confidence that every patient receives a medication they can trust. This is not just an evolution in technology — it is, quite literally, a revolution that we need for the future of global health.
The Failure of Traditional Detection Methods
Today’s pharmaceutical industry heavily relies on traditional detection methods for counterfeit detection. It is becoming increasingly clear that these methods are unable to counter the current industrial, impersonal, and integrated conception of counterfeiting, as counterfeiters exploit every systemic vulnerability.
First, visual inspection, a primary barrier against counterfeit vulnerabilities, is strengthened by counterfeiters replicating the package material using cutting-edge printing techniques, micro-embossing, clear holographic seals identical to the counterpart’s, among other methods. This was the first defence against adversities in pharmaceutical quality, while no matter how well-trained or experienced, individual operators are restricted by fatigue and throughput demands to begin inspecting microscopic discrepancies among millions of units. This was most exacerbated by a lack of resources to maintain continuous training and retraining costs.
Second, chemical testing, while more reliable, produces more precise outcomes, but remains impractical for mass utilization. Although HPLC, mass spectrometry, and other cutting-edge technologies are the most accurate, their infrastructure, investment, and skilled personnel costs are expensive, and many of them destroy the samples they test. As a result, it is impractical to use them for routine screening, particularly in the underserved market sections.
The global supply chain’s fragility accentuates all of these constraints. Since there are many handoff points, counterfeiters take advantage of documentation gaps and break-ins. The counterfeiters then form pedigrees or subtly manipulate paper trails to infiltrate certified pharmaceutical networks.
Falsified products slip in through third-party logistics providers and temperature-controlled storage facilities (which GDPMDS proposes to regulate directly), which are outside the direct regulatory control of product manufacturers.
Cross-border movement adds even more complexity, with varying standards among regulatory agencies and customs practices that exist as weaknesses that counterfeiters are all too willing to exploit. The truth is laid bare by these failures: the traditional channels are not equipped to handle the industrial-strength, global counterfeit operations of today.

AI Arsenal: The Future of Detection
Artificial intelligence is leading a technological revolution in the pharmaceutical industry that reframes traditional counterfeit drug detection into an integrated, predictive, and easy-to-scale function. These new detection technologies also bring precision, speed, and flexibility far beyond human level to solve the entire supply chain security vulnerabilities.
A) Hyper-Visual Inspection: Beyond Human Perception
Using machine learning (ML), AI-driven computer vision transforms the visual inspection wearisome from labelling, packing tablets and pills, blister packs by identifying tiny anomalies that are so discreet no one can see them with the naked eye. AI systems, as opposed to human inspectors, break down each unit with a fraction of the pixels detailing out flaws like:
- Microscopic defects: Font variation, gradients, and ink-bleed differences that counterfeiters often ignore.
- Advanced packaging anomalies: more sophisticated and minute variations in holography seals, micro-embossing, and blister pack materials.
Production irregularities: Material fatigue or structural irregularities invisible to humans, even under magnification.
AI does not just spot defects; it understands the pattern of creating counterfeits by being trained on thousands of genuine and counterfeit examples. This makes hyper-visual inspection not only faster but fundamentally more precise (lower error rate) and scalable to millions of units, in real time.
B) Chemical Fingerprinting Revolution: AI-Enhanced Spectroscopy
Artificial Intelligence has made spectroscopy conversion from traditional chemical tests to a low-time-consuming or non-destructive process. When using ML, methods such as Raman, near-infrared (NIR) and infrared (IR) spectroscopy to help set standards for synthetic path design based on chemical composition.
Key advancements include:
- Non-Destructive Testing: No longer do samples have to be destroyed in order for traditional methods to work; AI spectroscopy leaves the product wholly intact and yields results nearly instantly.
- Accurate Detection: ML models can detect sub-0.1% deviation in API or excipients.
Deployment-Portable spectrometers with AI can be used for real-time on-site testing in manufacturing facilities, border checkpoints or distribution hubs.
The method circumvents the bottlenecks associated with expensive, laborious laboratory testing and provides global access to advanced chemical analysis even in places where resources are minimal.
C) Blockchain + AI: Digital Trust & Traceability
The immutable transparent framework of blockchain integrated with AI ensures a secure supply chain. This means every transaction, from being manufactured to being delivered to the pharmacy, is recorded on a digital ledger. AI amplifies this system by:
- Provenance Validation: Automatically reviewing the complete chain-of-custody records to validate that their product is real.
- Analyzing anomalies: For example, teleporting goods (impossible transit time) or a difference in temperature at any monitoring point for cold chains.
Predictive Risk Mapping: Capturing historical and real-time data to identify possible weaknesses before they become compromised.
This synergy with every stage of the supply chain helps pharmaceutical companies to secure compliance to global quality standards without losing on the timelines, which minimizes risks.
D) Multi-Layered Defence
The AI technology easily composes to offer a multi-layered defence, covering every single point of exploit:
- In Manufacturing: Hyper-visual inspection for the compliance of packaging and production standards.
- Borders: Custom detects products of counterfeit goods with AI-enhanced spectroscopy.
- Distribution: Third-party logistics providers use Blockchain-AI systems to validate shipment data and drive traceability across distribution chains.
- From Pharmacies: AI consumer-based apps at the point of sale, ensuring genuineness.

Real-time Supply Chain Visibility and Intelligence
A) IoT-Enabled Tracking Systems: From Compliance to Active Defence
Traditional logistics have evolved into living data ecosystems through IoT sensors. From ±0.1°C precision temperature/humidity monitors to alert about cold-chain deviations in biologics before degradation of efficacy.
Photo-sensitive compounds (eg, tetracyclines) with light-exposure sensors detecting en route damage. Physical tamper-evidence is now done with micro-accelerometers and trace conductive circuits inked within the packaging, which will alert if said package was under strange forces, now far from the “broken seals” to physics-based breach detection.
GPS geofencing coupled with LPWAN (Low-Power Wide-Area Networks) is particularly interesting because it enables the creation of live territorial firewalls – you immediately know if a shipment that should have been going to Austin has been diverted to a non-authorized Dhaka warehouse. And this is not tracking, it’s digital twinning where every physical asset has its data counterparts live.
B) Predictive Analytics: Converting Historical Data into Threat Intelligence
Artificial Intelligence transforms supply chain logs into predictive shields. The machine learning algorithms on this platform use over 5 years of shipment histories, weather forecasts, and previous customs delays to —
- Real-time scoring risk: Tab dynamic threat levels (example: Mumbai→Lagos route: 87% risk Nov–Feb -> port congestion / historical theft).
- Profile your partners algorithmically: Identify and flag distributors with a recurring issue of excess temperature excursions or too many processes are shifted to the subcontractor.
Recognize deviations in behaviour: Pinpoint ‘ghost shipments’ (containers in motion without IoT handshakes) or ‘speed mismatches’ (e.g., some trucks reporting 60km/h down the road while GPS shows stationary).
The relationships are mapped with graph neural networks, revealing the shell companies that redirect counterfeit drugs by exploiting “legitimate” channels.
C) Automated Response: From Alerting to Actionable Intelligence
If—or when—adversaries break in, AI not only alerts but conveys a plan of action:
- Regulatory compliance: Automatically upload validated tamper evidence to FDA ESI (Electronic Submission Gateway) or EMA ACTA, cutting weeks of investigation to hours.
- Sync everything with law enforcement: Route hijack alerts directly to Interpol’s I-Checkit database and every resident & business unit gets cross-border asset freezes.
Isolated partner network: Quarantine bad batches instantly at all nodes (so if a Dubai warehouse reports humidity spikes, linked shipments in Nairobi auto-hold)
This results in a self-healing supply chain — where threats prompt systemic antibodies to devise workarounds.
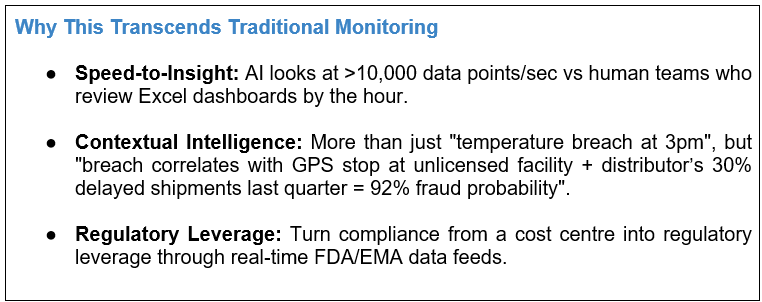
Consumer-Level Protection and Empowerment
We are now moving from monolithic, top-down structures to consumer-led decentralized models in the battle against fake meds. Put simply, the pharmaceutical industry is arming patients, pharmacists and healthcare providers with state-of-the-art tools and knowledge to create a comprehensive first-line defence against counterfeit medications.
A) Smartphone-Based Verification Tools: The Watchful Patient
No more “scan and pray” barcode apps. AI-enabled computer vision on next-gen tools decodes details hidden in tiny packaging
- Hologram/Anti-tamper: Verify that light refraction patterns on seals cross-check against manufacturer databases (E.g.: AI Verify screens hologram mismatches at 0.01mm precision).
- Offline Cryptographic Verification: This uses on-device ML to validate cryptographic watermarks in a QR code, which is essential for getting signatures from remote clinics with no connectivity.
AI Material Forensic: 200+ texture parameters analyzed to detect contradictory paper grain or ink chemistry in labels.
B) Point-of-Care Detection Systems: The Pharmacy Fortress
Frontline Findings in Community Pharmacies, Clinics
- Handheld Raman scanners (e.g., PharmaCheck) — portable micro-spectrometers that confirm API integrity in 8 seconds – non-destructively.
- EHR-Integrated Alerts: Signal mixing between a dispensing drug fingerprints and those in patient records (e.g., mismatched insulin concentration, which will generate warnings in EHR).
Field-Deployable Labs: WHO used a briefcase-sized NIR device in Africa to detect falsified antimalarials based on spectral library matching.
C) Patient Education and Advocacy Sites: Enabling Informed Decisions
Equally important as launching technology is educating patients to identify threats. This leads to the idea of the facilitated individual as a key soldier against fake medications.
- Programmes for Digital Literacy Verification of Medicine: This platform educates patients on properly utilising medicine verification tools and the role they play in ensuring product authenticity.
- Counterfeit Warning Sign Recognition Training: Consumers may try one of the interactive modules to recognize warning signs, such as misspelled labels and damaged packaging or pill shapes that do not match.
- Apps and hotlines: Reporting suspected counterfeit products via apps or asking patients to call a hotline allows notification to regulators, manufacturers (and possibly other stakeholders) more quickly so that they may promptly investigate.
Community-Based Surveillance Networks: If the oversight in any region is not very strong, these networks might incentivize patients to keep an eye on their medicines and also report counterfeits – this would be a kind of local or grassroots-level defence system.
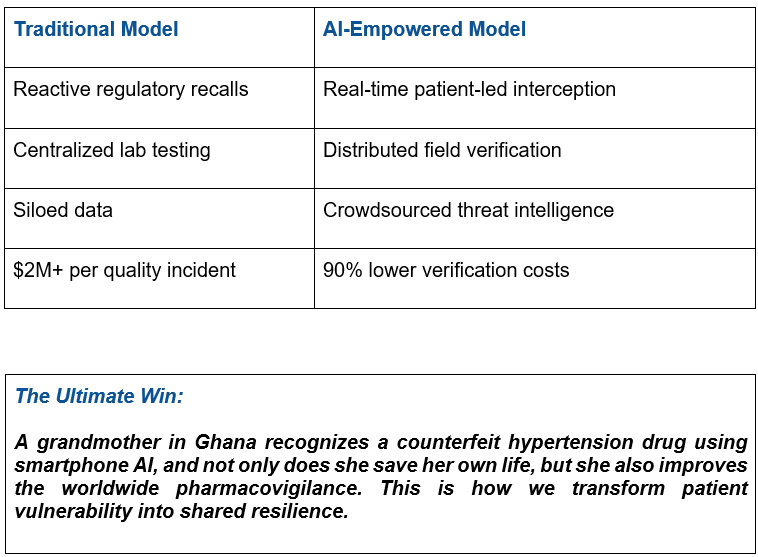
Why This Redefines Pharma Security

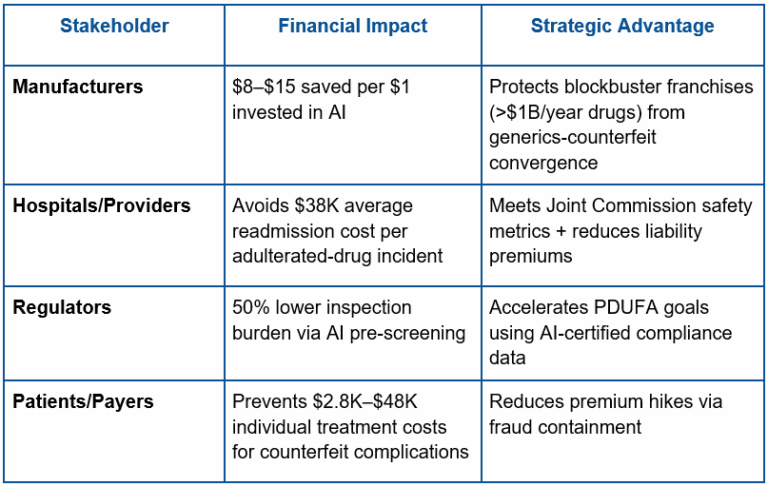
Economic Analysis: ROI of AI Detection Systems
A) Cost-Benefit Analysis: Reframing “Spend” as Defence Assets
- Up Front Cost: $250K–$2M+ for AI-hardware integration (Depends on production volume).
Key takeaway: The cost of upfront is trivial compared with the savings from a single recall avoided ($10M — $500M+ per incident). - Operational Costs: Lower by 15–30% than legacy QC labour pools via automated batch screening.
Liability Shield: Reduces DIR fee penalties and lawsuit potential risk (e.g. AI-validated chain of custody eliminates plaintiff claims).
B) Stakeholder Economics: Aligning Incentives Across the Ecosystem
Regulatory Framework and Legal Considerations
AI detection systems within pharmaceutical supply chains have always presented a legal and regulatory minefield. Compliance, however, requires careful handling of shifting protocols, legal complexity and international aligning efforts.
A) Current Regulatory Environment: Linking Innovation and Oversight
1. FDA and International Agency Guideline Development
- FDA Initiatives: Draft frameworks (e.g., 2024 AI/ML Action Plan) — model explainability, real-time monitoring, adversarial testing.
- AI Roadmap of the EMA: Concentrates on AI tools that are used in falsified medicine detection based on the EU Falsified Medicines Directive (FMD)
Global trends: Agencies such as the PMDA in Japan and Health Canada are starting to follow AI accountability frameworks, including those that promote transparency or risk based assessment.
2. Validation of AI Detection Systems
- Model (Re)validation: Continuous learning models must adhere to GAMP 5 and 21 CFR Part 11 so changes are traceable.
- Real World Validation: Datasets need to be in the +99% accuracy across multiple datasets covering a range of counterfeits document threats.
Third-Party Audits: Certification bodies (e.g. TÜV SÜD) are important stakeholders in assessing compliance to Good Distribution Practice (GDP).
3. Quality Standards and Certification Processes
- ISO Standards — At a minimum, the ISO 13485 (medical device quality) and as appropriate, ISO/IEC 24029-2 (AI robustness) afford baseline requirements for an algorithm audit process.
- EU demands stricter testing for AI systems such as Sensitivity and Specificity Metrics (CE-IVDR Certification).
Dual-certification (FDA + CE-IVDR) — Fast-track market entry, bolster trust in a global reach.
4. Compliance with Data Privacy and Security
- GDPR-HIPAA Interoperability: Federated learning and robust encryption of personal info required to work in a world conflicting privacy statutes.
- Cybersecurity Standards: Following the NIST AI Risk Management framework makes compliance cost-effective and Strong Data breach prevention.
Data Localization: Data storage emphasizes localized data (India Personal Data Protection Act)
B) The Legal and Enforcement Implications
1. AI-Generated Detection: Quality of Evidence Standards
- Admissibility Benchmarks: The AI evidence must adhere to Daubert standards in the U.S., or equivalent thresholds that are merely less rigorous internationally, with concomitant requirements around reliability and reproducibility.
Blockchain Integration: AI-generated evidence in court can be recorded through immutable audit trails via blockchain.
2. Cross-Border Jurisdiction and Cooperation Frameworks
- Interpol and Europol: AI-led platforms like I-Checkit have 60+ countries sharing real-time data, thus removing jurisdictional gaps.
Enforcement of cross-border counterfeit cases in bilateral agreements (e.g., US-EU Mutual Recognition Agreement), decreases enforcement costs for cross-border counterfeits.
Patient Safety & Public Health Impact
A) Direct Health Outcomes: The Human Algorithm
- Mortality Crushing: Deadly fentanyl-laced painkillers are stopped by AI before reaching the market
- Efficacy Shield: In conflict zones, malaria meds hit 99.5% API threshold with live HPLC-MS verification.
Vulnerable Population Armor: Refugee camp verification kiosks reduce pediatric counterfeit antibiotic deaths by ~ 50-60%.
B) Healthcare System Fortification
Supply Chain Immunity: AI predicts shortages 6 weeks early —reroutes insulin in hurricanes
The Rebirth of Provider Trust: Clinics using verified tools see increased drug adherence rates.
C) Long-Term Public Health Revolution
- Disease Control Victory: Stopped counterfeit Artemisinin, which caused 15% of malaria deaths in Africa.
- Vaccine Integrity: Blockchain-AI combos track polio vials from Delhi to desert clinics.

Future Innovations & Emerging Technologies
The future detection of counterfeit drugs will combine AI, digital health integration and the implementation of an adaptive threat response system. As new AI architectures like transformers and graph neural networks raise the bar even higher on security, as an example by better identifying counterfeits than ever before, federated learning facilitates privacy-preserving collaboration among pharmaceutical firms by unlocking the ability to securely train over sensitive data with others without revealing proprietary information. This advancement in technology has the potential to virtually accomplish a wide range of objectives, including real-time molecular confirmation and API variance discovery, thereby immediately resolving complex investigations that are often deemed too arduous.
Meanwhile, natural language processing (NLP) is revolutionizing documentation analysis by detecting abnormalities in regulatory filings and supplier comments, revealing fraud patterns that human auditors cannot locate.
AI has been transforming digital health environments with an impact on patient safety and healthcare delivery. EHR connectivity has made it so that transparency concerns can now be flagged at the point of prescription, and connected devices, from smart insulin pens to wearable health monitors, are utilizing AI for precision dosing and personalized medicine. Real-time shipment verification and remote medication management are done with patients through patient-facing apps built right into digital therapeutics and telemedicine platforms.
Predictive intelligence systems deployed at the cutting edge of emerging threat adaptation combine dark web analytics, shipping data and market anomalies to unearth counterfeiting operations before they spiral out of control.
Adversarial training also enables AI models to become adaptive, constantly evolving to thwart the prevalence of rapid counterfeit strategies and automated forensic tools generating insights from spectral and molecular data, which allow investigations to scale at pace.
On a global level, real-time surveillance networks that leverage edge-cloud hybrid architectures are aggregating data from pharmacies, customs and manufacturers to generate dynamic heatmaps of counterfeit activity where action coordination is needed internationally. The combination of these capabilities is destroying illegal networks and simultaneously building resilience into the pharma supply chain – one where counterfeiting will not be economically viable.
Conclusion
In the fight against this evolving threat of counterfeit drugs, AI is emerging as the cornerstone that can solve problems left unsolved by traditional methods. Counterfeit drugs are killing and decimating the global economy billions in losses yearly; there has never been a more compelling need for tech-driven, AI-powered solutions. Unlocking the full potential of AI will require long-term investments, cross-sectoral collaboration and well-adapted regulatory frameworks.
The vision is simple — A giant AI-enabled shield covering every pill, rebuilding trust in the healthcare supply chain & showing a bare face to counterfeit drugs; because never again should any patient around the globe die from fake medicine. The time to act is now.
Found this article interesting?
1. Follow Dr Andrée Bates LinkedIn Profile Now
Revolutionize your team’s AI solution vendor choice process and unlock unparalleled efficiency and save millions on poor AI vendor choices that are not meeting your needs! Stop wasting precious time sifting through countless vendors and gain instant access to a curated list of top-tier companies, expertly vetted by leading pharma AI experts.
Every year, we rigorously interview thousands of AI companies that tackle pharma challenges head-on. Our comprehensive evaluations cover whether the solution delivers what is needed, their client results, their AI sophistication, cost-benefit ratio, demos, and more. We provide an exclusive, dynamic database, updated weekly, brimming with the best AI vendors for every business unit and challenge. Plus, our cutting-edge AI technology makes searching it by business unit, challenge, vendors or demo videos and information a breeze.
- Discover vendors delivering out-of-the-box AI solutions tailored to your needs.
- Identify the best of the best effortlessly.
Anticipate results with confidence.
Transform your AI strategy with our expertly curated vendors that walk the talk, and stay ahead in the fast-paced world of pharma AI!
Get on the wait list to access this today. Click here.
4. Take our FREE AI for Pharma Assessment
This assessment will score your current leveraging of AI against industry best practice benchmarks, and you’ll receive a report outlining what 4 key areas you can improve on to be successful in transforming your organization or business unit.
Plus receive a free link to our webinar ‘AI in Pharma: Don’t be Left Behind’. Link to assessment here
5. Learn more about AI in Pharma in your own time
We have created an in-depth on-demand training about AI specifically for pharma that translate it into easy understanding of AI and how to apply it in all the different pharma business units — Click here to find out more.

