What Is Living Intelligence in AI and Why Does It Matter for the Future?
In the rapidly changing world of pharmaceuticals today, AI systems that were revolutionary 2 years ago have promptly become out of date as so much changes in that time. Regulatory authorities are accelerating approvals for breakthrough therapies, while pharma players are saddled with inflexible old models that cannot keep up with shifting clinical data complexity. The industry’s staggering $2.3B average cost to develop a single drug isn’t merely a capital problem, it’s an indicator of a larger issue-the absence of adaptive intelligence.
Existing static algorithms, designed for static information, work well with past patterns but fail to keep pace with the real-world variation of the pharma world today – changing regulations, multi-variable manufacturing processes, and increasing heterogeneity of drug targets. What the industry requires is not simply improved models but a new way of thinking: Living Intelligence – leveraging AI technology that’s dynamic, context-sensitive, and capable of evolving in response to new biology breakthroughs and process anomalies. The debate is no longer how to use AI but how quickly can you transition from static algorithms to adaptive intelligence before your pipeline gets left behind the competition.
Living Intelligence: Beyond AI to Adaptive Systems
Understanding Exponential vs. Linear Technological Advancement
Within pharma, legacy systems – and mindsets – overwhelmingly reflect linear growth expectations. For years, advances in drug development followed well-defined, step-wise paths: incremental improvement in discovery, formulation, trials, and manufacturing. However, the current era is characterized by exponential technological advancement, where each innovation builds on the last at an accelerating rate. This shift isn’t just theoretical – it’s disrupting the foundational economics and operating models of pharma.
Linear systems optimize for control and consistency but fail in environments of rapid change and complexity. Exponential technologies, on the other hand, don’t just move faster – they change the rules altogether. This is vital in an industry such as pharma, where variables in biology, chemistry, regulatory landscapes, and global supply chains interact in unpredictable ways. Traditional AI methods – those trained once and deployed statically – often reflect linear thinking. They assume a stable environment.
Living intelligence embraces exponentiality: adapting, recalibrating, and learning from living data in real time, in sync with the pace of biological and technological discovery.
The Three Converging Forces Driving Living Intelligence
The emergence of Living Intelligence in pharma is not due to one breakthrough but the convergence of three powerful technological domains. These forces are not operating in isolation – they are colliding, reinforcing and amplifying each other. This convergence represents a foundational shift in how pharmaceutical value is created, optimized, and defended.
1. Advanced AI: The Computational Engine
At the core of living intelligence lies a more sophisticated form of artificial intelligence – not just trained on retrospective datasets, but continuously retraining itself on streaming data, contextual signals, and real-world outcomes. These models are no longer bounded by static training epochs. They are designed to evolve, to interpret out-of-distribution events, and to reconcile conflicting inputs dynamically.
What separates this class of AI from prior generations is its tech stack architecture and its operational philosophy. It’s not about prediction alone; it’s about cognition under uncertainty. In pharma, where variables such as patient response, batch quality, or environmental impact can deviate from the norm at any moment, these AI systems act less like calculators and more like decision-making co-pilots. They can identify latent patterns, flag causal anomalies, and suggest course corrections mid-stream – whether in a clinical trial protocol or a biologics fermentation run. This ability to interact with live data and adapt accordingly redefines what computational models can deliver to both research and operations teams.
2. Sensor Proliferation: Creating Digital Nervous Systems
Data integrity has always been a limiting factor in pharmaceutical innovation. Theoretical models, no matter how advanced, are only as good as the data feeding them. This is where the explosion of sensing technologies becomes transformational. From lab environments to commercial production lines, from patient wearables to cell culture monitoring systems, sensors are creating real-time, high-resolution streams of data that capture the full spectrum of variability.
These sensors are not just passive recorders – they are the foundation of a digital nervous system. They allow every component of the drug development and manufacturing lifecycle to be continuously observed, analyzed, and optimized. Instead of relying on batch-level data, organizations can now respond to second-by-second fluctuations in process parameters, reagent interactions, or patient feedback.
In clinical settings, sensor networks offer unprecedented visibility into patient adherence, metabolic response, and behavioural patterns – creating data ecosystems that blur the line between clinical trial and real-world evidence. In manufacturing, inline sensors reduce lag between detection and intervention, significantly decreasing quality risks. This live instrumentation of the environment is what gives AI the contextual awareness needed for living intelligence.
3. Bioengineering: Integrating Organic and Inorganic Intelligence
Bioengineering is redefining the relationship between human biology and engineered systems. The most profound breakthroughs are occurring at the interface – where biological processes are being measured, influenced, and even co-processed by intelligent systems. This isn’t about replacing human insight; it’s about embedding it into materials, devices, and workflows.
On the discovery side, bioengineered platforms enable high-throughput experimentation that mimics physiological conditions more accurately than ever before. Cell systems, organoids, and microfluidic models generate data that is richer and more biologically relevant, allowing living intelligence systems to learn in vitro what would previously have required costly and lengthy in vivo trials.
Moreover, biologically-integrated systems are enabling adaptive feedback loops – where patient data, molecular performance, and manufacturing variables inform each other in real time. A therapy’s design, testing, and production are no longer siloed steps; they become fluid phases of a single adaptive system. The combination of biology’s adaptability with machine-scale precision is allowing organizations to reimagine both the speed and specificity of pharmaceutical development.
How These Technologies Amplify Each Other: Compounding Effects in Practice
Each of the three pillars – advanced AI, sensor proliferation, and bioengineering – delivers value on its own. But their true potential is revealed in how they amplify one another through feedback loops and shared data substrates. Sensors generate high-fidelity, real-time inputs. Bioengineered systems contextualize those inputs in biological relevance. Advanced AI models integrate and interpret those inputs dynamically. And the outputs from these models feed back into design or manufacturing decisions, which are in turn monitored by sensors – completing the loop.
This creates compounding effects. Improvements are no longer isolated – they cascade. A marginal gain in sensor resolution leads to a jump in model accuracy. A small leap in bioengineering yields more meaningful training data for AI. Over time, these accelerations multiply – not arithmetically, but geometrically – reshaping the entire pharmaceutical value chain.
This is the essence of the technology supercycle: not just more powerful tools, but a fundamentally different operating model. One where intelligence is not fixed, but alive – fluidly integrating the synthetic and organic, the algorithmic and empirical. Pharma leaders who embrace this convergence will not merely improve efficiency – they will redefine competitiveness.

Why One-and-Done AI Approaches Fall Short
The pharma sector’s over-reliance on static, single-point AI solutions on a single set of data is an unseen crisis, injecting systemic risks into the pipeline of pharmaceutical development. These are not technical faults but structural flaws that are exacerbating risks in the era of personalized medicines, adaptive regulatory mechanisms, and biological complexity.
1. The Brittle Foundations of Static AI
Legacy AI deployments often fail under three overlapping pharma-specific stresses:
- Temporal Fragility: Models that are trained on past data fail to absorb emergent in vivo responses, post-marketing surveillance patterns, or abrupt shifts in disease epidemiology (e.g., viral escape mutations making antiviral candidates outdated mid-study).
- Regulatory Rigidity: Hard-coded rules cannot self-calibrate to shifting ICH/FDA evidentiary requirements and hence generate submission packages that adhere to yesterday’s regulatory requirements but not tomorrow’s.
- Biological Blindspots: Deterministic paradigms view human physiology as closed system, with significant drug mechanism-patient microbiome, epigenetic terrain, or tumour microenvironment-adjustment interactions unrepresented.
The cascades of consequences are these: lead molecules designed by AI with perfect in silico blueprints fail at Phase II as they are subject to actual biological dynamism, whereas process conditions optimized based on stationary PAT restrictions experience batch failure due to variability in raw material beyond agreed limitations.
2. The Law of Diminishing AI Returns
Conventional AI generates logarithmic value decay across the therapeutic lifecycle:
- Discovery Phase: Early wins in target identification (40-60% efficiency gains) plateau as models ignore newly discovered off-target interactions visible only through CRISPR-edited organoid assays.
- Clinical Development: Fixed patient stratification algorithms miss biomarker-defined responder subgroups detectable via continuous wearable/sensor streams, forcing costly protocol amendments.
- CMC: Quality control AI trained on historical batch data falters when novel excipient interactions emerge during scale-up, triggering stability failures.
This decay pattern creates a perverse innovation tax—organizations expend disproportionate resources retrofitting obsolete models rather than advancing novel therapies.
3. The Silo Trap: Strategic Paralysis by Design
Siloed AI architectures institutionalize three fatal disconnects:
1. R&D ↔ Manufacturing: Perfect target profiles with no manufacturability constraints in terms of feasibility offer molecules for unrealistic synthesis processes (e.g., cryogenic conditions unrelated to commercial-scale bioreactors).
2. Clinical ↔ Commercial: Trial designs blind to real-world dosing patterns produce therapies with adherence barriers invisible to clinic-controlled administration.
3. Regulatory ↔ Research: Submission strategies divorced from live regulatory intelligence lead to avoidable clinical holds—e.g., failing to pre-emptively address emerging FDA concerns about class-specific cardiovascular risks.
The Living Intelligence Imperative
The industry’s inflection point demands a paradigm shift from:
- Static → Adaptive Systems: Architectures where algorithms evolve with real-time in situ biomarker streams, adaptive trial protocols, and self-calibrating manufacturing controls.
- Silos → Synapses: Neural networks connecting preclinical toxicology data to post-market AE reports, turning pharmacovigilance from damage control into predictive science.
Deterministic → Probabilistic Models: Frameworks that treat disease states as probability clouds rather than fixed targets—critical for addressing cancer heterogeneity or neurodegenerative disease progression.
Economic and Productivity Transformation
In pharma, this means output is increasingly driven by intelligent systems, not headcount. R&D is improving not with added resources but through smarter prioritization and adaptive experimentation. Manufacturing efficiencies result from real-time feedback rather than hardware upgrades.
Beyond Molecules: Value from Intelligence
Pharma’s future value will not come from new compounds alone but from ongoing optimization. Real-time adjustment of therapy, tracking of outcomes, and adaptive regulatory strategies are now critical levers.
Data itself—when repeatedly honed and contextually deployed—becomes a strategic asset. This creates new revenue models based on adaptive systems rather than drug sales alone.
The convergence economy opens up 5 never-before-seen opportunity vectors:
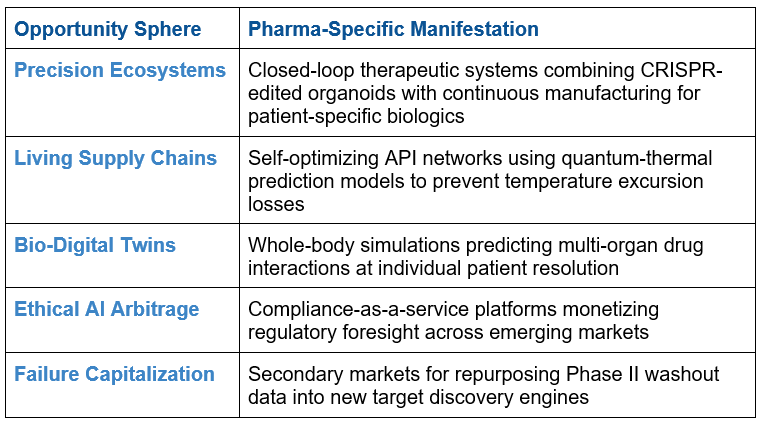
Building Living Intelligence Organization-Wide
1. Evaluating Current Capability and Determining Gaps
Pharmaceutical organizations need to shift beyond rigid digital maturity models and instead measure adaptive capability—how fast insights move through the value chain and affect decisions. That means looking at “data metabolism”—how fast late-stage insights inform discovery or how quickly manufacturing variations drive changes in formulation.
Another critical measurement is decision latency, particularly for clinical and manufacturing processes. Agile organizations allow front-line teams to act within bounded systems with less reliance on hierarchical escalation.
A key diagnostic is the “insight half-life”—the time it takes for a safety message or discovery insight to affect downstream processes. A high half-life indicates fragmented intelligence and requires structural change, not just surface-level fixes.
2. Designing Cross-Functional Intelligence Architectures
Future-ready pharma companies build intelligence systems that function like a nervous system—integrated, adaptive, and continuous. Three architectural principles are essential:
- Reciprocal knowledge streams, where downstream learnings reshape upstream decisions (e.g., manufacturability driving molecule design).
- Dynamic feedback loops, allowing real-time quality or safety adaptations based on live data, not static SOPs.
Cross-domain translation, turning patient outcomes into design constraints or clinical profiles into manufacturing variables.
This calls for an exchange of siloed centers of excellence with cross-functional teams—combining scientific, engineering, and regulatory talents toward common therapeutic objectives. Without semantic and strategic alignment, shared data alone cannot drive unified decisions.
3. Living Intelligence Data Infrastructure
Living intelligence demands infrastructure that replicates biological systems: adaptive, contextual, and continuously evolving. Traditional data lakes fall short. What’s required is stratified knowledge systems that link molecular interactions to clinical outcomes, integrating genomics, imaging, assays, biomarkers, and real-world data.
Dynamic ontologies need to change with new science—reshaping relationships when novel interactions or safety signals are discovered. Systems need to enable both real-time (synchronous) decisions (e.g., adapting process controls) and long-term (asynchronous) learning (e.g., safety insights impacting discovery). Incorporating design-by-regulation principles makes compliance a natural aspect of what’s created—rather than a secondary consideration—enhancing both innovation and documentation integrity.
4. Talent Strategies for the Age of Living Intelligence
The talent model must transition from specialization to systems fluency. Future pharma professionals are hybrid thinkers: biologists with data science skills, analysts rooted in biology, and regulatory professionals who are algorithm thinkers. Organizations need to place more emphasis on systems thinkers—those who natively link molecular behaviours to clinical or regulatory results. Internal ecosystems should encourage deliberate collisions between disciplines to drive innovation. Without this integrative talent strategy, companies will end up creating siloed expertise incapable of providing cohesive intelligence or driving therapeutic progress.

Implementation Roadmap: From Vision to Reality
A) Strategy first!
A comprehensive AI strategy serves as the foundation for pharmaceutical companies navigating the complexities of AI implementation. Rather than pursuing disconnected pilot projects, this strategic framework ensures AI initiatives directly support broader organizational objectives.
Strategic alignment delivers value across multiple dimensions. It directs resources toward high-impact opportunities like accelerating drug discovery timelines, streamlining clinical trial operations, and advancing personalized medicine approaches. This focused investment approach maximizes return while minimizing scattered efforts.
The strategic framework also promotes organizational integration by encouraging cross-departmental collaboration and eliminating data silos that often hinder AI effectiveness. When departments work cohesively, data flows seamlessly throughout the organization, enabling more powerful AI applications.
Additionally, a well-structured strategy provides a clear implementation pathway. This includes building essential infrastructure, recruiting specialized talent, and developing internal capabilities necessary for sustainable AI adoption. Without this roadmap, organizations often struggle with ad-hoc implementations that fail to scale.
Most importantly, embedding ethical considerations, regulatory compliance, and data security into the strategic foundation protects the organization while building stakeholder confidence. This proactive approach to governance prevents costly missteps and establishes trust that’s essential for long-term AI success in the heavily regulated pharmaceutical industry.
B) Starting Small After Strategy: Pilot Projects with Measurable Outcomes
After creating a solid strategy that identified the optimal places to begin, implementation begins with focused pilots in high-impact areas for measurable, real-world outcomes.
In pharmacovigilance, adaptive signal detection can cut latency from weeks to near real-time through the integration of spontaneous reports, trial safety data, and EHRs.
During the trial phase, pilot biomarkers should focus on self-optimizing stratification models, redefining success in terms of the speed at which such systems are improving specificity and sensitivity.
In manufacturing, adaptive control systems should streamline batch quality in real-time—especially for biologics- through parameterization adjustment by product characteristics and dramatically reducing variability. These pilot runs need to occur within closed systems, where comparative traditional practices give reference points upon which gains in performance are measured.
C) Scaling Strategies: Beyond Proof-of-Concept
Scaling necessitates systematic scaling beyond the primary use case. Cross-therapeutic scaling requires first to take successful models—such as adaptive trial models—from one disease area to others and scale for clinical and biological relevance.
Second, vertical scaling calls for systems qualified in early trials to develop into later stages with built-in regulatory readiness, like auto-generated compliance reports.
Third, geographical scaling must localize adaptive tools to varied regulatory and clinical contexts. Leading organizations simulate agency expectations through “regulatory twins” prior to broader deployment, ensuring that scalability aligns with compliance and operational readiness rather than mere technical potential.
D) Integration Approaches: Breaking Down Technological Silos
Living intelligence relies on bridging data and functional silos. Integration should begin with harmonizing data across domains through cohesive ontologies—linking molecular, clinical, manufacturing, and commercial data based on shared semantics.
Next, validate hypothesis-to-market context propagation: learning gained through discovery needs to inform clinical development, manufacturing specifications, and market strategy.
Lastly, facilitates real-time feedback loops where post-market insight can influence decisions up-stream. Integration needs to support both structured and unstructured data through NLP engines trained in pharma-specific vocabulary, with middleware that translates across functional vocabularies to facilitate continuous cross-domain learning.
E) Measuring Success: New KPIs for the Era of Living Intelligence
Legacy metrics fall short in adaptive systems. New KPIs include:
- Decision Latency: Latency between signal emergence and action (e.g., trial adaptation or process adjustment).
- Knowledge Recirculation: Rate at which downstream insights (e.g., real-world data) affect upstream activity.
- Failure Intelligence Utilization: Percentage of failed assets or deviations that provide predictive gains.
Compound Intelligence Half-Life: The duration that insights from determinated efforts still influence decisions.
These KPIs need to be institutionalized—embedded in governance, made visible in dashboards, and linked to incentives. Blindly following traditional stage-gate metrics can cause misalignment and slow genuine transformation.
Ethical Imperatives and Regulatory Navigation
Privacy in Sensor-Rich Environments
As pharma makes the leap to real-time data capture with wearables, implantables, and environmental sensors, privacy concerns are piling up. Wearables and implantables collect sensitive, granular information—beyond the point of informed consent in most cases. To address this, firms need to:
- Apply differential privacy at the edge to disentangle clinical signals from lifestyle patterns.
- Utilize dynamic consent frameworks, enabling participants to have control over the use of their data during the study.
Deploy regional privacy engines to process cross-border data while adhering to local regulations.
Trust & Transparency in Adaptive Systems
As adaptive systems shape clinical and manufacturing choices, transparency is mandatory. Best practices are:
- Implementing decision provenance infrastructure to track how, why, and when algorithms are modified.
- Providing algorithm explainability, especially for patient-confrontational applications, with peer-reviewable logic.
Placing adaptive systems as continuously evolving scientific tools—not black-box technology to build trust and prevent regulatory backlash.
Complexity Management in Regulations
Legacy regulatory practices conflict with adaptive systems. Pharma must shift towards continuous compliance, such as:
- Predictive adaptation ranges for algorithm upgrades within pre-tested ranges.
- Performance corridors permitting real-time adaptations without periodic revalidation.
- Regulatory digital twins for simulating compliance effects before implementing modifications.
Transition from static reports to dynamic evidence packages that evolve with the system.
Ethical Frameworks for AI in Pharma
Adaptive intelligence needs to be steered by health effects-specific ethics. This entails:
- Counterfactual testing to establish improved outcomes compared to usual care.
- A harm detection system to identify non-optimal algorithm drift early on.
- Transparency and user control over clinical choices.
- Bias audits and equity committees to ensure fair performance across groups.
Ethical circuit breakers that can halt systems nearing ethical limits.
In pharma, ethics is not a compliance add-on; it’s a design principle. If it is not treated as such, it can jeopardize trust, reputation, and regulatory setbacks.
Conclusion
The pharmaceutical industry is in the midst of a fundamental transformation as living intelligence – self-adaptive, perpetually adaptive systems that reshape drug discovery, development, manufacturing and commercialization into a continuous, neural-like environment. The shift promises more than efficiency; it provides real-time responsiveness, predictive accuracy and faster timelines, requiring not only tech revamps but also a cultural and leadership transformation.
Success depends on breaking down silos, integrating cross-functional data fluidity, and adopting ongoing, emergent decision-making. Early adopters will establish new standards in the industry through rolling regulatory models, bespoke manufacturing, and adaptive candidate-to-clinic movement, building a competitive advantage that rigid systems can’t deliver. Eventually, those who blend technology, biology, and analytics into an integrated learning entity will define the next wave of pharmaceutical innovation—where responsiveness, not traditional expertise, defines lasting value.
Found this article interesting?
1. Follow Dr Andrée Bates LinkedIn Profile Now
Revolutionize your team’s AI solution vendor choice process and unlock unparalleled efficiency and save millions on poor AI vendor choices that are not meeting your needs! Stop wasting precious time sifting through countless vendors and gain instant access to a curated list of top-tier companies, expertly vetted by leading pharma AI experts.
Every year, we rigorously interview thousands of AI companies that tackle pharma challenges head-on. Our comprehensive evaluations cover whether the solution delivers what is needed, their client results, their AI sophistication, cost-benefit ratio, demos, and more. We provide an exclusive, dynamic database, updated weekly, brimming with the best AI vendors for every business unit and challenge. Plus, our cutting-edge AI technology makes searching it by business unit, challenge, vendors or demo videos and information a breeze.
- Discover vendors delivering out-of-the-box AI solutions tailored to your needs.
- Identify the best of the best effortlessly.
- Anticipate results with confidence.
Transform your AI strategy with our expertly curated vendors that walk the talk, and stay ahead in the fast-paced world of pharma AI!
Get on the wait list to access this today. Click here.
4. Take our FREE AI for Pharma Assessment
This assessment will score your current leveraging of AI against industry best practice benchmarks, and you’ll receive a report outlining what 4 key areas you can improve on to be successful in transforming your organization or business unit.
Plus receive a free link to our webinar ‘AI in Pharma: Don’t be Left Behind’. Link to assessment here
5. Learn more about AI in Pharma in your own time
We have created an in-depth on-demand training about AI specifically for pharma that translate it into easy understanding of AI and how to apply it in all the different pharma business units — Click here to find out more.

