3D printing has revolutionized manufacturing across industries and around the world. The deceptively simple concept of “additive manufacturing”, as it’s also called, involves laying down layer upon layer of material to produce three-dimensional objects of varying complexity. Today, it is being used to create everything from organs for transplant to toys, weapons, and even bridges.
3D printing is a far more sophisticated process than, say, grinding up chemicals and pressing them into a pill shape, or creating a liquid solution of active and supporting ingredients and encapsulating them in gel. And that’s an exciting prospect when it comes to drug delivery, a process that hasn’t changed all that much in the last several hundred, even several thousand years (our word for ‘pill’ comes from the latin Pilula, coined by Pliny in the 1st century AD, in reference to small, compact doses of medicine meant to be ingested).
3D printing, and the unprecedented level of control it offers over drug manufacturing and delivery, has widespread implications for the future of the pharmaceutical industry, both medically and commercially.
In this article, I discuss the game-changing potential of 3D printing medicine, and what it means for patients, practitioners, and manufacturers.
Personalized medicine, on-demand
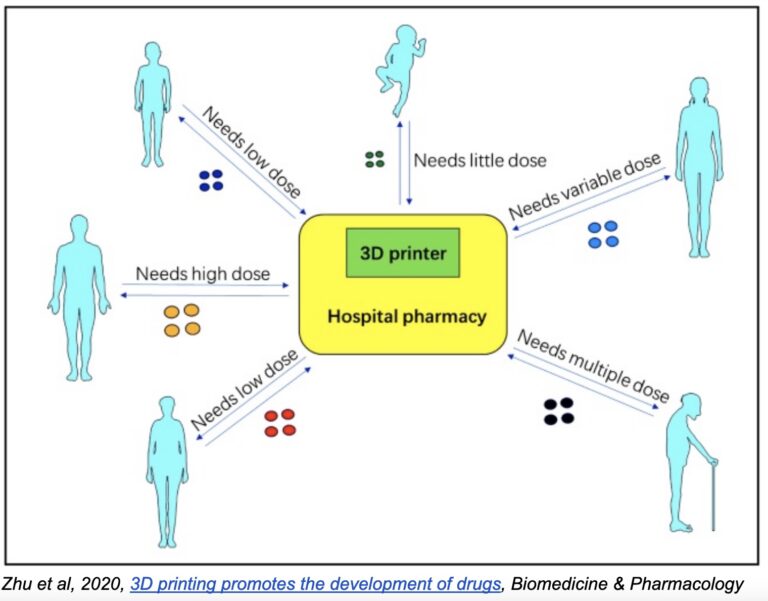
One of the most exciting—and revolutionary—aspects of 3D drug printing is the ability to finely control the physical arrangement of active and inactive ingredients within pills. The organization and density of these ingredients, as well as the overall shape of the pill, determine in large part the pill’s release and absorption rates.
Thus, by making small adjustments to a standardized model, pills can be manufactured with precise release and absorption rates based on individual needs—something sure to have an enormous impact on the future of personalized medicine. Imagine patients going to the pharmacy and picking up a prescription of pills designed specifically for them, based on their unique physiology, biology, and disease stage.
Of course, the same goes for dosage. Dosages are standardized across pills for operational reasons: assembly-line production methods don’t allow for variability in dosage or pill size. It’s just not practical or cost-effective to produce a wider range of dosages. But with 3D printing, the process is quite the same whether you’re printing 15mg, 18mg, 23mg or 60mg. The volume of material changes, and the printing time may be slightly longer or shorter, but in practice, any dosage can be created at basically zero additional operational cost.
3D printing can also be used to make pills that are easier or more comfortable to swallow. Aprecia’s ZipDose® Technology, for example, has enabled it to create highly porous, rapidly disintegrating pills that are simpler to administer and swallow—a boon for patience with e.g. epilepsy. This is a big step forward in patient adherence, which has been positively linked to ease of administration and swallowing.
Custom polypills likewise improve adherence and patient experience, especially for individuals burdened with multiple medications for chronic and age-related diseases. Consider, for example, that many cancer patients take 5, even 10 pills per day. Again, operational difficulties once precluded the possibility of making polypills for each individual patient, but with 3D printing, it becomes much easier and cost-effective.
Of course, 3D printing of drugs is a new technology, and there is still much to be learned about the process and outcomes. In one recent trial of 3D polypills printed using a technique known as stereolithography, the authors discovered that one of the component drugs, amlodipine, interacted with the photopolymers used in the printing process, rendering it inert.
‘Our study demonstrated for the first time that the drug and the photopolymer could possibly undergo unexpected reactions, which is clearly undesirable. This interaction was a Michael addition, which happens in mild conditions without the use of any catalysts or solvents.’ — Simon Gaisford, University College London.
Nonetheless, the potential for cost-effective, practical personalization is revolutionary.
Printers at hospitals, doctor’s offices… and homes?
One of the most important consequences of 3D printing has been the democratization of manufacturing. Small in size and relatively inexpensive, 3D printers dramatically reduce the cost of producing a variety of items, both household and exotic. Plus, a single printer can produce an almost infinite number of objects. All that’s required is a digital model and the right materials.
Now that democratization is coming to pharmaceuticals. One way this may come to pass is with pharmaceutical companies providing hospitals and doctor’s offices with the precursors necessary to manufacture their own pills. Again, the implications for individualized medicine are staggering.
But it may also lead to significantly reduced costs in unused medication (patients are prescribed exactly the number of pills they need, and no more or less, or printed individualized polypills), storage (precursors are likely to take up far less space than packaged pills), and more.
Another way 3D printing may be integrated into hospitals and doctor’s offices is through chemical microreactors—miniature versions of chemical reactors that, when fed simple precursors, can produce an enormous variety of different medicines. This was demonstrated by a team of researchers, whose paper published in Science (Kitson et al, 2018), demonstrates how they were able to 3D print microreactors capable of producing the muscle relaxant baclofen, an anticonvulsant, and a drug to fight ulcers and acid reflux.
The implications for rural hospitals and treatment centers are especially exciting. With limited storage and budgets, these institutions have had to historically pick and choose what medications they keep on hand. Being able to produce their own pills would go a long way in improving patient outcomes in remote areas.
There are, of course, significant regulatory concerns with such an approach, though. Moving drug production from the highly-controlled, regularly-audited environment of manufacturing plants to hospitals and, perhaps down the road, doctor’s offices, is a thorny issue. The FDA’s initial Technical Considerations for Additive Manufactured Medical Devices already speaks to many of these issues, and foretells a complex regulatory environment.
Early efforts to ensure the quality and safety of printed materials are promising, though. These efforts are well summarized in a 2022 article in The Pharmaceutical Journal:
“For example, researchers have explored the use of real-time analytical technologies, such as near-infrared and Raman spectroscopy, to ensure the quality and safety of formulations produced using 3D printers. Others have suggested using ‘track and trace’ measures by including QR codes and data matrices on formulations that can be scanned using a smartphone device or barcode readers to ensure drug product quality and safety. Others have highlighted the potential for use of a blockchain to track printed formulations and increase safety[103]. Additionally … several companies are developing GMP-compliant 3D printers that can be appropriately cleaned and validated to ensure printed drug product safety and quality.”
In some perhaps not-so-distant future, we may even one day see the presence of tightly regulated, in-home printers for specific chronic conditions. Cancer patients, for example, may be able to procure precursors and a specific digital model allowing them to print their own polypills from home. But the ethical and regulatory issues here are complex, and the potential for abuse cannot be overlooked.
Implications for pharmaceutical companies
Individualisation on a massive scale, microscale production of drugs in hospitals and doctor’s offices—these represent big changes to the industry and have important implications for pharmaceutical companies.
For one, they shift some of the onus of production onto many, individual operators, rather than large-scale production plants owned and operated by pharmaceutical companies (although such plants will still be responsible for supplying molecules in the form of precursors). At least, that is unless (until?) printed microreactors become more widespread (whether legally or clandestinely).
There is a potential for abuse and lost profits, though. 3D printing has already been used for illegal purposes, like producing weapons or reproducing patented objects, and it seems inevitable these misuses will spread to drug manufacturing. Counterfeit medicines are already a huge problem for both the pharmaceutical industry and patients, and 3D printing is unlikely to alleviate the problem.
Nonetheless, there are extraordinary benefits here for patients, practitioners and pharmaceutical companies. 3D printing of drugs is here and very likely here to stay.
Conclusion
The pharmaceutical industry must prepare for these changes, seize opportunities to improve patient outcomes through individualized medicine, and work with practitioners and regulators to ensure its deployment is done safely and securely.
Found this article interesting?
Tune in to our podcast ‘AI for Pharma Growth‘, episode 24 ‘How to print drugs‘ in which Dr Andree Bates interviews Alvaro Goyanes, the Founder and CEO of FabRx.

FabRx is a specialist biotech company, focused on developing 3D printing technology for fabricating pharmaceuticals and medical devices. Using patented technologies, they provide a platform for the production of personalised medicines, revolutionising medical treatment for patients around the world. Alvaro believes the future of medicine is in 3D printing. His aim is to 3D print medicines in hospitals in order to create personalised medications. Find out about the future of drug manufacturing and the future delivery of personalized medicines in this episode.
Listen on Apple here.
https://podcasts.apple.com/us/podcast/e24-how-to-print-drugs/id1616728442?i=1000577850261
Listen on Spotify here
https://open.spotify.com/episode/2MkK3r0FXn8IKU7AmVzs6w
For more information, or to be a guest on the show, contact Dr Andree Bates abates@eularis.com.

